The key to an atom’s chemical characteristics is its electron
configuration. This configuration determines the kinds and
number of bonds an atom will form with other atoms.
The Formation of Bonds with Carbon
Carbon has 6 electrons, with 2 in the first electron shell and
4 in the second shell; thus, it has 4 valence electrons in a
shell that holds 8 electrons. A carbon atom usually completes
its valence shell by sharing its 4 electrons with other atoms
so that 8 electrons are present. Each pair of shared electrons
constitutes a covalent bond (see Figure 2.12d). In organic
molecules, carbon usually forms single or double covalent
bonds. Each carbon atom acts as an intersection point from
which a molecule can branch off in as many as four directions.
This ability is one facet of carbon’s versatility that
makes large, complex molecules possible.
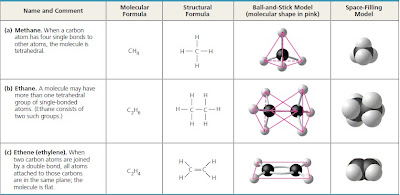
When a carbon atom forms four single covalent bonds,
the arrangement of its four hybrid orbitals causes the bonds
to angle toward the corners of an imaginary tetrahedron (see
Figure 2.17b). The bond angles in methane (CH4) are 109.5°
(Figure 4.3a), and they are roughly the same in any group of
atoms where carbon has four single bonds. For example,
ethane (C2H6) is shaped like two overlapping tetrahedrons
(Figure 4.3b). In molecules with more carbons, every grouping
of a carbon bonded to four other atoms has a tetrahedral
shape. But when two carbon atoms are joined by a double
bond, as in ethene (C2H4), the atoms joined to those carbons
are in the same plane as the carbons (Figure 4.3c). We find it
convenient to write molecules as structural formulas, as if the
molecules being represented are two-dimensional, but keep
in mind that molecules are three-dimensional and that the
shape of a molecule often determines its function.
The electron configuration of carbon gives it covalent compatibility
with many different elements. Figure 4.4 shows the
valences of carbon and its most frequent partners—hydrogen,
oxygen, and nitrogen. These are the four major atomic components
of organic molecules. These valences are the basis for
the rules of covalent bonding in organic chemistry—the
building code for the architecture of organic molecules.
Let’s consider how the rules of covalent bonding apply to
carbon atoms with partners other than hydrogen. We’ll look at
two examples, the simple molecules carbon dioxide and urea.
In the carbon dioxide molecule (CO2), a single carbon
atom is joined to two atoms of oxygen by double covalent
bonds. The structural formula for CO2 is shown here:
O“C“O
Each line in a structural formula represents a pair of shared
electrons. Thus, the two double bonds in CO2 have the same
number of shared electrons as four single bonds. The arrangement
completes the valence shells of all atoms in the molecule.
Carbon atoms can form diverse molecules by bonding to four other atoms
Maret 28, 2013
1